THE RIGHT THERAPY
FOR EVERY PATIENT
RENAL ACCESS
Across our current HD and PD catheter portfolio, there is a long history of clinical performance and several unique features designed to promote best in class outcomes.
A PORTFOLIO WITH BEST IN CLASS OUTCOMES
Chronic Hemodialysis
Our Palindrome™ Precision chronic dialysis catheters are currently the only catheters on the market with two types of coating technologies — anti-thrombogenic and antimicrobial technologies — combined on a single catheter to address two of the primary complications with long term dialysis catheters.
Acute Hemodialysis
Our Mahurkar™* acute hemodialysis catheters include our proprietary laser-cut side slot design (also part of our Palindrome™ Precision line) to provide next-generation blood handling capabilities to reduce the risk of clot formation by minimizing blood component adhesions.
Peritoneal Dialysis
Our Argyle™* peritoneal dialysis catheters offer one of the most comprehensive PD catheter lines on the market to provide a tailored catheter configuration for each patient.
Fistula Access
RCS Canada also offers AV Fistula Access products including the Argyle Fistula Cannula as well as the WingEater, SysLoc Mini and Harmony fistula needles manufactured by JMS.
MAHURKAR™* ELITE
ACUTE HEMODIALYSIS CATHETER
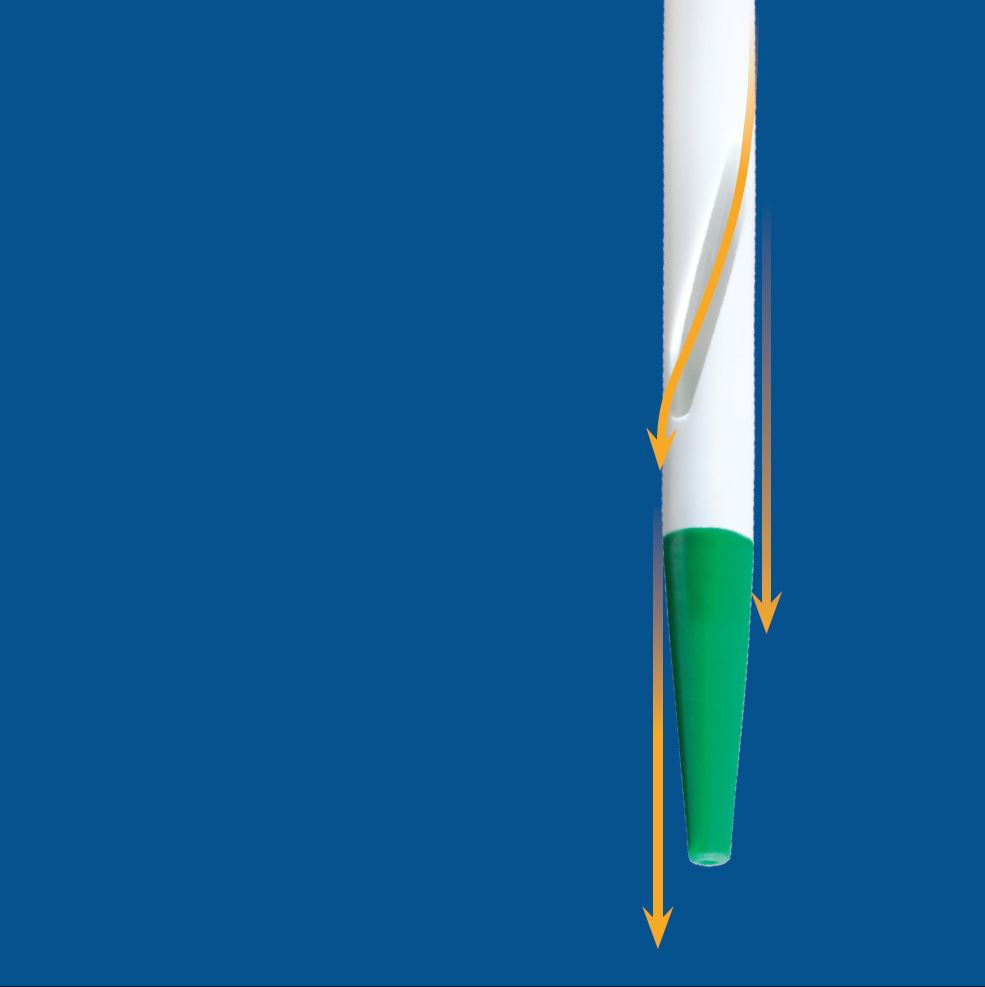
A CATHETER TIP DEVELOPED
WITH DIALYSIS EFFICIENCY IN MIND
Mahurkar™* Elite LARGE LASER-CUT SIDE SLOTS:
- Reduce the likelihood of vessel wall occlusion by maximizing the passageway for fluid flow1
- Have smooth edges to decrease the likelihood of clot formation
Mahurkar™* Elite SYMMETRICAL TIP DESIGN:
- Leads to low recirculation in forward and reverse flow2
- Includes a soft atraumatic tip
1 Mahurkar Elite Product Family Design Validation Test Report 50136-172-MS-11, Sept. 30, 2013
2 Mahurhar Elite 12Fr Dual Lumen Acute Catheter Design Verification Test Report 50136-155-MS-11, Project No. 2.17.18.10, Oct 2, 2012
PALINDROME™ PRECISION
CHRONIC
HEMODIALYSIS CATHETER

Did you know that poor tip position accounts for 20% of early catheter removal?1
According to 2019 KDOQI guidelines, the proper location of the CVC tip is at the mid right atrium.2
What is the impact of catheter tip design on accurate placement?
Different catheters have different functional tips* that will determine ease of positioning. Functional Tip placed too high or too low in the SVC or SVC/RA Junction can create unwanted results and complications.3
The symmetric tip of the Palindrome™ Precision catheter has a more compact functional tip compared to other tip designs, allowing more flexibility in positioning the catheter tip within the right atrium.
* Functional Tip: Part of the catheter from the most proximal side hole to the catheter tip.
1 Trerotola, SO. Hemodialysis Catheter Placement and Management. Radiology 215: 651-658, 2000.
2 KDOQI CLINICAL PRACTICE GUIDELINE FOR VASCULAR ACCESS: 2019 UPDATE.
3 Tal M, Freedman T, Mojibian H. Dialysis Catheter Tip Placement: The Functional Tip. A different look at a contentious topic. Endovascular Today, June 2013.
Did you know the incident use of Tunneled CVC in Canada is 80%?1
Although the preferred access for hemodialysis is the radiocephalic native vessel arteriovenous fistula, the use of tunneled central venous catheters continues to increase in most Canadian hemodialysis units.
Catheter long-term complications include catheter thrombosis, central vein stenosis, fibrin sheath formation, and catheter-related infections.2
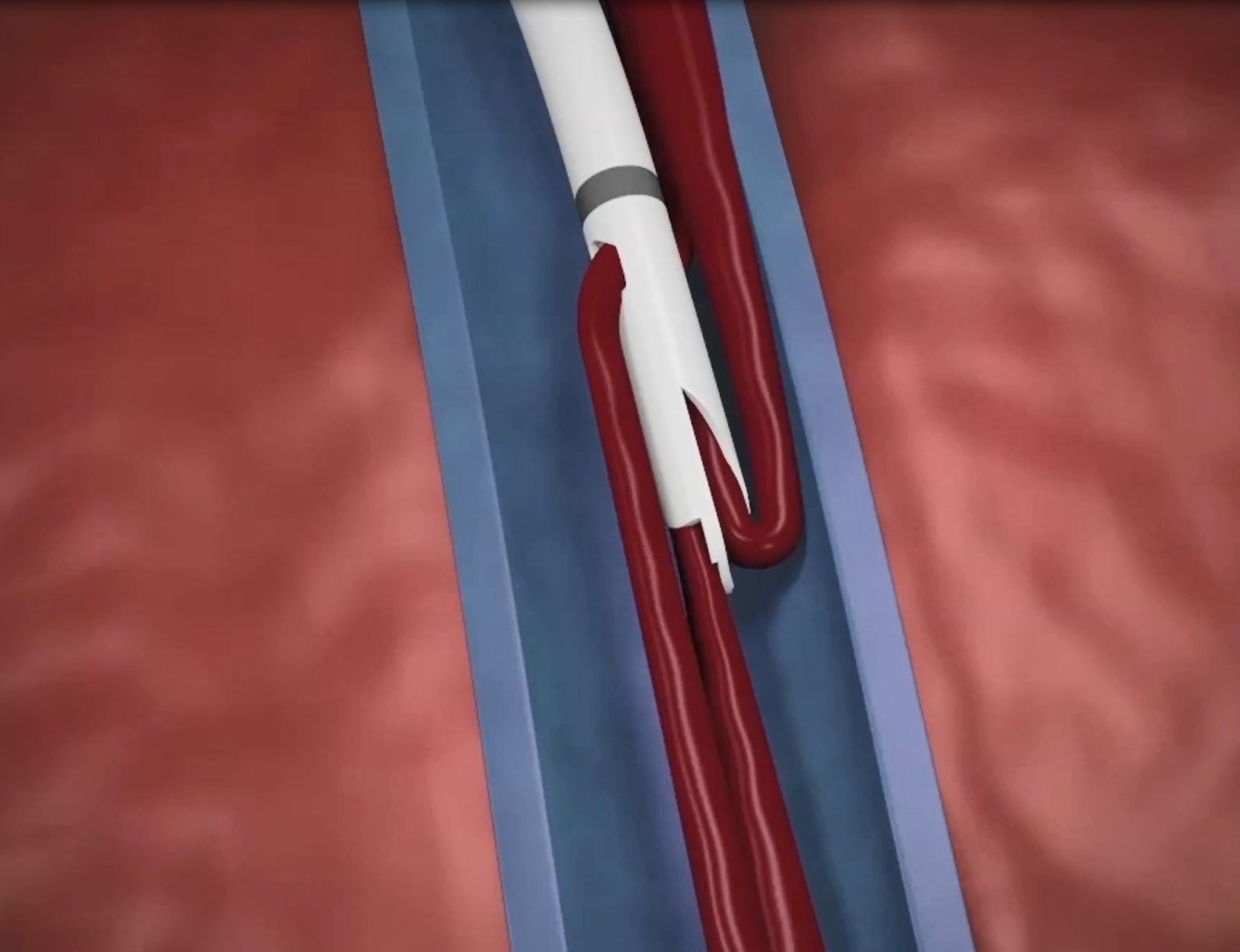
The Palindrome™ Precision Dialysis Catheter has been developed to help reduce catheter complications. The large laser-cut side slots combined with the symmetrical tip design create an environment with a reduced potential for thrombus formation.3
1 Canadian Society of Nephrology. Report of the Canadian Society of Nephrology Vascular Access Working Group. Semin Dial. 2012;25(1):22-25.
2 Source: Canadian Organ Replacement Register, 2012 Canadian Institute for Health Information
3 Spector, M, Mojibian H, Eliseo D, Clinical outcome of the Tal Palindrome chronic hemodialysis catheter. J Vasc Interv Radiol, 2008;19 (10):1434-1438.
ARGYLE™* PERITONEAL DIALYSIS CATHETER

Catheter solutions to face your peritoneal dialysis challenges
Manufactured with hand-made quality for over 20 years, the Argyle™* family of peritoneal catheters offers a wide variety of catheter options to help you meet the clinical challenges of your peritoneal dialysis patients. Argyle™* peritoneal catheters are made of soft, flexible and radiopaque silicone. The catheters are available with one or two felt cuffs which create firm catheter anchors into the tissue and reduce the likelihood of bacterial invasion of the peritoneal cavity.
Pediatric PD catheter configurations & accessories
Medtronic offers a wide variety of pediatric peritoneal catheter configurations and lengths to meet the needs of all of your pediatric patients from infant to adolescent.
Many peritoneal catheter accessories are also available to to help you meet the catheter placement and maintenance needs of all of your patients.
ARGYLE™*
FISTULA CANNULA
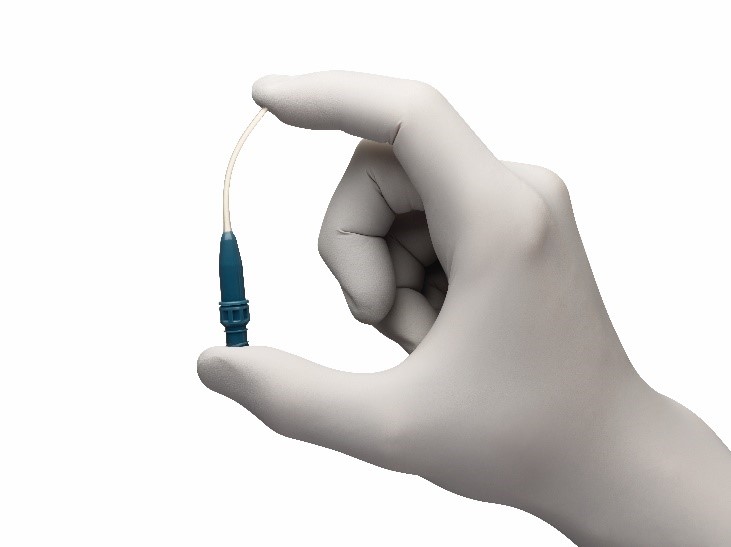
Did you know...
Cannulation is one of the primary causes of AVF complications and failure?1,2
Cannulation technique is an important aspect of long-term AV access patency. Each blood vessel puncture may incite local trauma and subsequent venous neointimal hyperplasia and stenosis formation.3 Infiltration alone is a significant complication, with one study reporting an annual major infiltration rate of 5.2%, and concluding that one infiltration leads to three extra months of catheter dependency.4
Fistula Cannula with anti-reflux valves
The Argyle™* Fistula Cannula with anti-reflux valve enhances clinician safety and patient comfort. The plastic material is more flexible than a steel needle and it flexes to accommodate tortuous vessels and deep access. Studied in comparison to a steel needle, the plastic Argyle™* Fistula Cannula reduced the likelihood of hematoma.5 In simulation, the plastic cannula did not infiltrate the AVF/AVG wall as compared to a steel needle, which damaged the artificial wall.6
1 The End-Stage Renal Disease National Coordinating Center (ESRD NCC) Fistula First Catheter Last (FFCL) Workgroup Coalition. Fistula First Initiative: AVF–the first choice for hemodialysis. Available at: http://fistulafirst.org/. Accessed December 2, 2015.
2 Lee T, Ul Haq N. New developments in our understanding of neointimal hyperplasia. Adv Chronic Kidney Dis. 2015;22:431-437
3 Hsiao JF, Chou HH, Hsu LA, et al. Vascular changes at the puncture segments of arteriovenous fistula for hemodialysis access. J Vasc Surg. 2010;52(3):669-673.
4 Lee T, Barker J, Allon M. Needle infiltration of arteriovenous fistulae in hemodialysis: risk factors and consequences. Am J Kidney Dis. 2006:47:1020-1026.
5 Letachowicz K, Kusztal M, Golebiowski T, et al. Use of plastic needles for early arteriovenous fistula cannulation. Blood Purif. 2015;40:155-159.
6 Data on file. Argyle™ Fistula Cannula Patient Survey. 2015.
JMS
FISTULA
NEEDLES
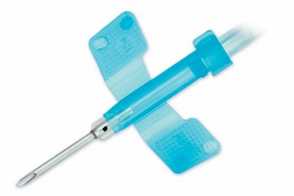
JMS Fistula Needles – Designed for safe and easy cannulation
The SysLoc®MINI combines innovative technology and the audible “Click” to ensure the needle is safely protected. The SysLoc®MINI can be operated with a one-handed or two-handed technique.
The WingEater® provides needle stick protection for a safe health care setting. The WingEater® fistula needle allows your facility to comply with OSHA guidelines on needle stick prevention.
The Harmony Fistula Needle is for use with a previously-established mature access site using a constant-site (buttonhole) technique of needle insertion. This Fistula Needle is specially designed for home dialysis patients and patients that self-puncture.
© 2021 Medtronic. All rights reserved. Medtronic, Medtronic logo and Further, Together are trademarks of Medtronic.™* Third party brands are trademarks of their respective owners. MAHURKAR™*, Double-D™*, and the Double-D design are U.S. registered trademarks of Sakharam D. Mahurkar, used under license. Argyle™* is a trademark of Cardinal Healthcase Inc., used under license. All other brands are trademarks of a Medtronic company. 04/2021 – CA-RCS-0044-E
